Chapter 15: Sex and Reproduction in Seed Plants
Seeds are a remarkable innovation that have been highly important to the evolution of plant life. The vast majority of plants that we observe and utilize possess seeds and seed plants dominate most terrestrial habitats. The last chapter described in general terms what seeds are and what modifications in the general plant life cycle of alternation of generations had to occur in order for seeds to appear. This chapter fills in some of the details for the five groups of seed plants:
- flowering plants, with 250,000 species, by far the most diverse and ubiquitous plant group
- conifers, although with only 1000 species, they are very commonly encountered and ecologically and economically important
- cycads, a small group of around 300 species with limited distribution and importance
- gnetophytes, a small group of only three genera and around 100 species
- Ginkgo, a single species that survives only where cultivated
In addition to these five extant groups, there are several groups of seed plants well represented in fossils but no longer present. These extinct species are sometimes lumped as ‘seed ferns,’ but both the lumping and the designation as a group are not thought to be accurate: they are not closely aligned with ferns and they probably represent an artificial (polyphyletic) grouping. Most workers believe that seeds evolved more than once and therefore that there should be no phylogenetic entity corresponding to either seed plants or to gymnosperms (i.e., seed plants lacking flowers) although these categories do persist. We will consider the details of the life cycle, in particular the form of the female gametophyte and the mechanisms associated with pollination and fertilization, for the five groups of extant seed plants.
TOPICS
- Conifer seed development
- Seed development in other gymnosperms
- Seed development in angiosperms
- Flowers
- Floral modifications
- Fruits
Conifers

Pines are the most commonly seen conifers and the group that will be described below but the basic pattern holds for all of the group. The plants that are recognized as pines are diploid, sporophyte plants. All conifers, including pines, are heterosporous and produce two kinds of spores, both on the same tree. The sites of spore production are the cones. The cones that most people recognize as ‘pine cones’ are female pine cones. These are not only the site of megaspore production but also the site of: megaspore germination that forms a gametophyte, egg production by that gametophyte, egg fertilization, and ultimately seed development. All these events take place in a location described as an ovule. These processes generally take multiple years and the structures one usually recognizes as pine cones have been living and developing over a time of two years or more, with many of the significant events occurring when the cone is much smaller and not as easily observed (Fig 1). A female cone consists of an axis (stem) bearing scales subtended by bracts, with the scales thought to be derivatives of modified branches. Upon the upper surface of the scale are the ovules, the structures that develop into seeds.

Male spores are produced in less familiar, but certainly easily observed, male pine cones, which grow more quickly than female cones but are present on the tree for a much shorter time. Typically, they are produced in the fall and are visible as a cluster of structures at the base of bud. These expand in the spring/early summer (Fig. 2) and dry up and wither a month later. In contrast to the female cones, m ale cones are simple in structure: a branch with tightly packed spore bearing leaves (sporophylls) , each with a pair of relatively large sporangia on their lower surface.
The gamet ophyte generation develops from microspores and megaspores that are produced and retained in the male and female cones. Gametophytes are highly reduced and, especially for the female gametophyte, largely invisible because of its small size and location. Male gametophytes are produced in the microsporangia of the male cones. These initially contain cells that undergo meiosis to produce microspores. At first, the spores are in clusters of four, reflecting their origin in the two divisions of meiosis (one cell to two cells to four cells). The spores eventually separate and undergo a very limited period of development, producing a haploid organism with four nuclei, usually in three cells (i.e., one cell has two nuclei), and possessing two wing-like air sacks (Fig. 3). Particularly significant to the development of the pollen grain (aka male gametophyte), is the fact that its development is arrested. This, and the fact that the microsporangium breaks open, allows the pollen to be dispersed by the wind.
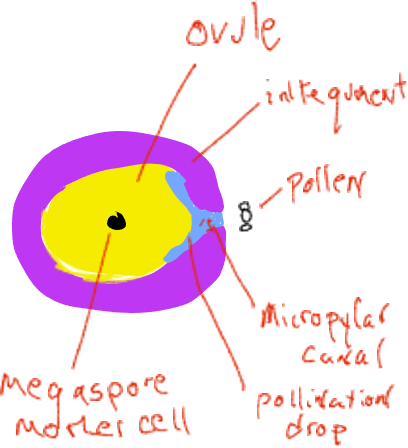
Pollination is the name of the transfer of the male gametophyte (pollen) from where it is produced to the location of the female gametophyte, in conifers, a movement from a male pine cone to a location inside the female cone. At the time of pollen release, female cones are very small and are ‘open’ with spaces above each individual cone scale that are open to the outside (Fig. 4). Each female cone scale bears on its upper surface two ovules, each with a megasporangium imbedded in sporophyte tissue called integuments. Early in ovule development there is an opening, the micropylar canal, between the integuments that connects to a space between the cone scales. Pollen grains (male gametophytes) in the air can slide between the female cone scales and be deposited in the space next to the micropylar canal. The ovule secretes a liquid ‘pollination drop’ into this space and pollen grains end up in the liquid and rehydrate. In a mechanism not completely understood, the liquid with the pollen grains is withdrawn through the micropylar canal to a space on the inside of the integuments adjacent to the megasporangium.
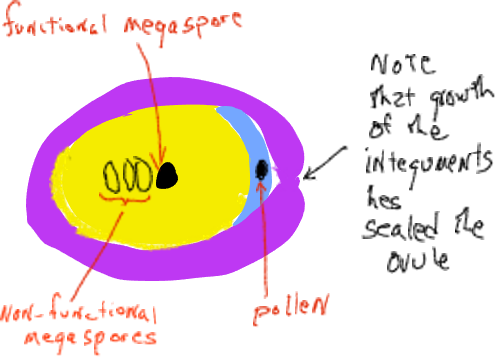
Soon thereafter the integuments grow to block the micropylar canal and the cone scales grow to seal the female cone off from the outside. At this time the megasporangium has a single megaspore mother cell destined to undergo meiosis. After meiosis, only one of the four daughter cells remains as a megaspore. The megaspore is not dispersed but develops within the megasporangium into a female gametophyte of several thousand cells that generally produces two or three archegonia, each of which produces a single egg. All of this occurs inside the female cone that is attached and is part of the sporophyte plant. The female gametophytes of conifers are highly reduced ‘organisms’ found within the ovulate cones, imbedded inside sporophyte tissue of the plant.
At the time of pollination there usually is no female gametophyte, only a megaspore. Inside the cone, the male gametophyte s continue their development, albeit very slowly in pines, requiring 12 months between pollination and fertilization.
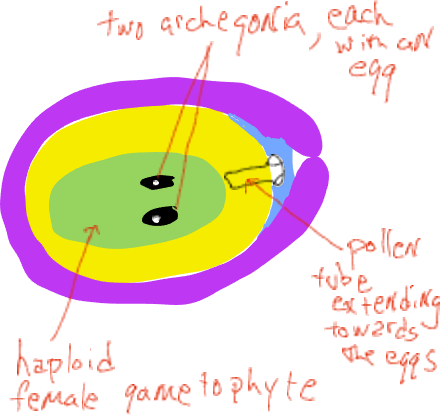
As the megaspore produces a spore that slowly develops into a female gametophyte, it goes through a ‘free nuclear’ stage where mitotic divisions are not accompanied by cell wall formation (the organism is coenocytic). Eventually cell walls form and the female gametophyte forms structures described as archegonia, each with a single egg.
As the female gametophyte develops, the pollen germinates and a single cell elongates from the grain, growing through the megasporangium (the nucellus) towards the female gametophyte (Fig. 6).. A little over a year after pollination this tube cell fuses with the egg cell and two sperm nuclei are released, one of which fuses with the egg nucleus, forming a zygote while the other nucleus disintegrates. During the time between pollination and fertilization, the female cones grow only a small amount and they remain closed to the outside. Note that no swimming sperm is produced, the male gametophyte grows to the egg by means of an elongate cell.
Following fertilization, the zygote develops into an embryo, imbedded in, and nourished by, the female gametophyte. Tissues surrounding the female gametophyte develop into a seed coat, often producing a wing structure that allows the seed to be dispersed by the wind. As the seed develops, the cone surrounding it also develops, often growing substantially. Following fertilization, seeds may mature in as short a time as one year but for most species it is two years or longer. In most pines, the cones eventually re-open, allowing the seeds to fall out and be dispersed by the wind. Sometimes the cones remained closed and only open following the intense heat of a fire. The female cones of junipers and yews develop fruit-like features that attract animals who facilitate seed dispersal by consuming the ‘fruits’ and defecating the seed in a new location.
Other gymnosperms
The three other groups of seed plants without flowers, Gnetophytes, Cycads and Ginkgo exhibit the same basic pattern of seed production: male spores develop into pollen grains which are dispersed from the sporophyte to finish their development in the structure that produces female spores and hence the female gametophytes. Pollination in at least some cycads and in some gnetophytes involves insects; in ginkgo and most gnetophytes pollination is by the wind. Gymnosperm literally means ‘naked seed’ and one feature that unifies the non-flowering seed plants is that, at the time of pollination, the ovules are accessible, not buried in tissues that the male gametophyte must grow through; instead, the ovules are available, at least for a brief period of time, because the cone scales have not fused with each other and the micropylar canal is open. However, the male gametophyte generally DOES have to grow through the megasporangium (the nucellus) to reach the egg. In cycads, the male gametophyte actually develops a type of feeding structure (called an haustorium), a branched filamentous structure permeating the nucellus and apparently obtaining nourishment from it. Eventually a flagellated, mobile sperm is released and swims through the fluid of an ‘archegonial chamber’, an area of fluid between the nucellus and the female gametophyte. Flagellated sperm are also found in ginkgo.
Flowering plants
The basic process of seed development in flowering plants is the same as in conifers. The major differences include the following:
- Male (pollen producing) and female (seed producing) organs are usually found together in the same structure, the flower, not separated on two distinct branches as they are in conifers.
- The ovules are produced inside a structure called an ovary, that is not open to the outside, thereby requir ing the male gametophyte grow through a substantial distance of sporophyte tissue in order to contact the female gametophyte
- The transfer of pollen (pollination) often involves biological agents (insects, birds, rarely mammals) and a variety of floral features enhance pollination.
- The female gametophyte , which is called an embryo sac (Fig. 7).
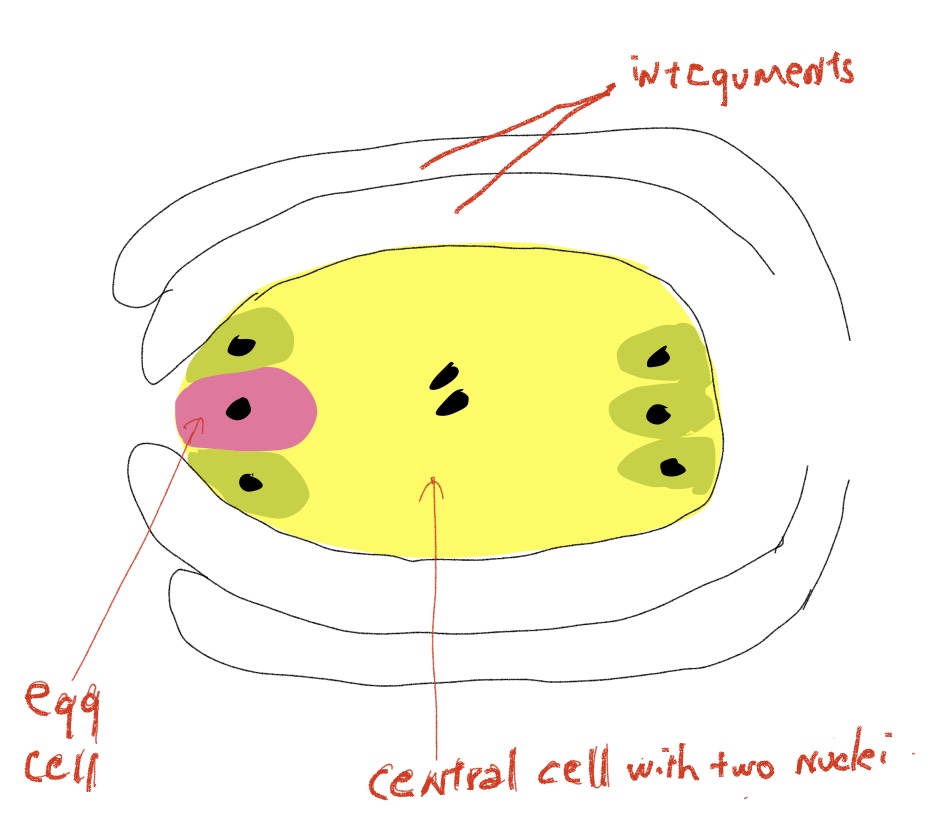
Fig. 7 An angiosperm ovule containing a mature female gametophyte (aka embryo sac). The gametophyte contains only seven cells, one an egg that gets fertilized to form a zygote and a large central cell that gets fertilized to form the triploid endosperm tissue. The surrounding integuments develop into the seed coat. because it eventually contains the embryo, is even more reduced than in other seed plants, drastically so, usually consisting of only seven cells, six haploid cells, one of which is the egg, and one larger central cell with two haploid nuclei.
- Both of the sperm nuclei produced by the male
gametophyte participate in a fertilization (syngamy) event. One fuses with the egg to form a zygote and the second fuses with the central cell. This sperm nucleus combines with the central cell’s two nuclei to form a triploid ‘endosperm’ nucleus. The central cell then proliferates, forming a tissue, endosperm, that has a limited development and is only found during seed development and often, but not always, in the mature seed. The initial stages of endosperm development involve a ‘free-nuclear’ stage where nuclei divide with no cell wall formation, creating a multinucleate, all triploid, (coenocytic) cell. This material is called ‘liquid endosperm’ and is familiar as coconut milk, which is actually cytosol. - In contrast with other seed plants, e.g., the conifers, in angiosperms the female gametophyte, which is very limited both in size and lifespan, is not the nutritive tissue for the developing embryo. In angiosperms the nutritive tissue for the developing embryo is the endosperm, the tissue resulting from a second fertilization event.
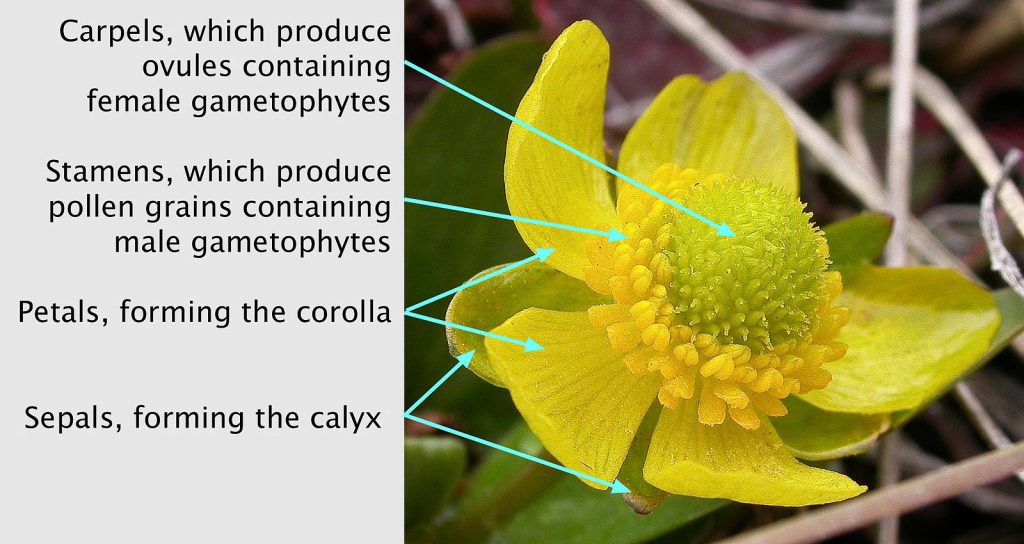
The flower
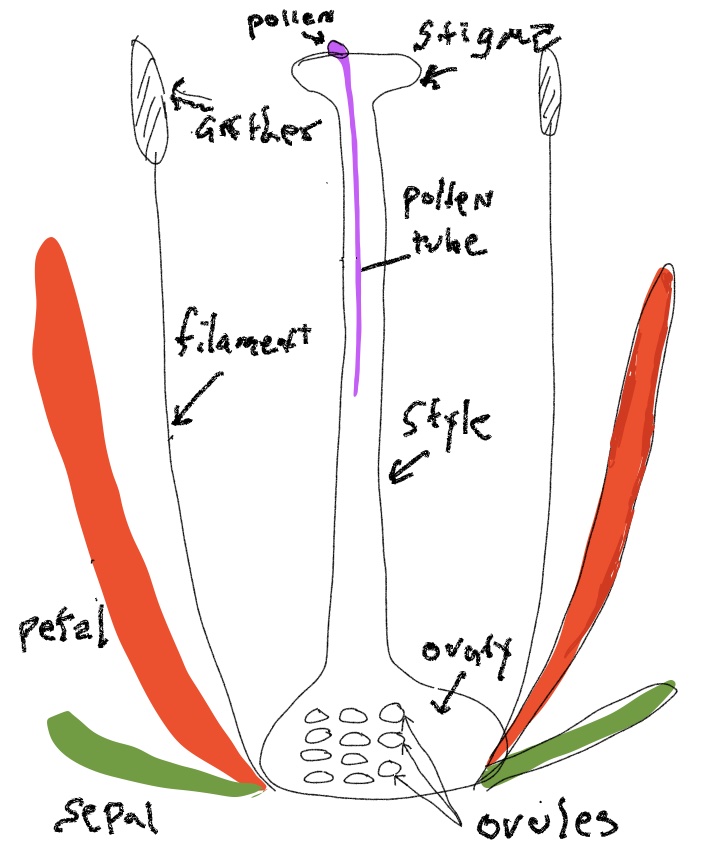
The flower is a highly modified stem, typically with four whorls that are bunched close together at the end of the branch. The components of each of the four whorls is thought to represent modified leaves, with the inner two whorls being highly modified spore bearing leaves (sporophylls) (Fig. 8-9). The elements of outermost whorl (sepals) are the most leaf-like, although often quite small. The elements of the next whorl (petals) are often leaf-like in form but usually are large and colorful structures that lack chlorophyll. The next whorl consists of stamens that often made up of of a stalk (a filament) terminating in a structure called an anther in which pollen is produced. Initially, the anthers possess microsporangia containing microspore mother cells. These produce microspores by meiosis and these spores germinate and develop into male gametophytes, aka pollen grains, composed of only two or three cells. When pollen is mature the anther generally opens up to make the pollen accessible to pollinators or the wind. Carpels are the innermost whorl and often are fused together so the central structure of the flower is a single (‘compound’) pistil. Carpel(s) generally consist of an enlarged base (the ovary), with a stalked structure (the style) emerging from its top that terminates with a surface (the stigma) that receives pollen. Inside the ovary are produced one too many ovules that eventually become seeds. Prior to this, the ovules are sites of megaspore production, female gametophyte (= embryo sac) development, fertilization and finally the process of seed development. Following pollination, the two-or-three celled male gametophyte (pollen) germinates on the stigma and grows through the style and then gains access to an ovule by growing through the micropylar canal, an opening between the integuments that surround each ovule. Pollen tube growth involves the expansion of a single cell, called the pollen tube, that delivers two male gametes to the embryo sac with the egg cell. The tube cell fuses to the embryo sac and delivers two sperm to bring about double fertilization, allowing for the subsequent development of the zygote and endosperm.
Floral modifications
The variations in the basic plan of flowers are one of the amazing stories of botany and of all biology. In general, the changes can be attributed to the forces of natural selection acting on the interaction between pollinators and plants. Some of the common transformations from the pattern described above include:
- fusion of the parts of a whorl, e.g., all the petals fused together to form a cup or funnel
- fusion of the members of two whorls (e.g., fusion of stamens on to the petals)
- reduction in the number of members of a whorl, in particular a reduction from many pistils to a single pistil as found in the flowers of Asteraceae flowers
- change from radial symmetry (all parts of a whorl being the same size and oriented in a similar fashion, Fig. 9-10) to bilateral symmetry with flowers having two sides that are mirror images of each other (Fig. 11), or sometimes to have no symmetry at all.
- placement of the ovary below the point of attachment of the other whorls
- elimination of multiple parts, sometimes forming unisexual flowers (Fig. 12).



The fruit
After fertilization, the ovule transforms into a seed in a process that involves the coordinated development of three distinct tissues:
- the zygote grows into an embryo;
- the endosperm proliferates, first in a ‘free-nuclear’ pattern (nuclear divisions are not accompanied by cell wall formation) and subsequently by producing new cells, and finally, in some groups, the endosperm disappears as the embryo enlarges its cotyledon(s);
- the tissues surrounding the embryo and endosperm develop into a seed coat.
Note again that the genetic makeup of these three components differs—the embryo is a ‘new generation’ and is diploid, the endosperm is triploid and the surrounding tissues are diploid but are of a generation before that of the embryo.
While the ovule transforms into a seed, the ovary, and sometimes other tissues surrounding the ovary, develop into a structure called the fruit. The transformation of the ovary into a fruit generally involves the production of new cells, the growth of these cells and the development of features specific to plant being observed. The fruit generally has features that enhance the dispersal of seeds and often has features that protect the seed. Although it is generally easy to distinguish the seed from the fruit, occasionally there is no obvious demarkation between them or there may be a demarkation that is deceiving. For example, almonds are derived from a fruit that is like a cherry (in fact cherries and almonds are very closely related). We eat the fleshy part of cherries, which is part of the fruit. The ‘pit’ is not actually a seed but rather it is the seed surrounded by the innermost layer of the fruit. When you ‘shell’ an almond you are cracking open fruit tissue to reveal a single seed inside. In this case the protective role of the seed coat has been taken over by a portion of the fruit (Fig. 13). Similarly, a sunflower ‘seed’ is actually a one-seeded fruit and shelling the ‘seed’ is actually splitting open the fruit (Fig. 14).


Further Reading and Viewing
- “The Botanist in the Kitchen” by Katherine A. Preston. Great site with a variety of excellent essays.
- Plant of the week forest service posts.
- “Alsomitra macrocarpa – High Fliers” by Scott Zona. Winged seeds (also search for Alsomitra macrocarpa).
- “The ancestral flower of angiosperms and its early diversification” by Hervé Sauquet. The ancestral flower of angiosperms.
- “Asphodelus aestivus” by Tamara Bonnemaison. Nice site that is not posting new photos but has nice archive. Botany flower of the day.
- “Apple Growth and Crop-load Management” by Steve McArtney. Apple fruit growth.
Media Attributions
- Pine pollen © Jon Houseman is licensed under a CC BY-SA (Attribution ShareAlike) license
- labeled flower © Matt Lavin is licensed under a CC BY-SA (Attribution ShareAlike) license
- Almond ‘nut’ © Mandel is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
- Sunflower seed © Kaldari is licensed under a Public Domain license

