Part IV: Pleistocene Epoch
18. Paranthropus boisei
Paranthropus boisei (2.5 mya)
(“beside human” / Boise: surname of one of the Leakey’s financiers)
SITES
Ethiopia: Shungura Deposits and Konso
Kenya: Chesowanja, West Lake Turkana, and Koobi Fora
Tanzania: Olduvai and Peninj
PEOPLE
Mary and Richard Leakey
INTRODUCTION
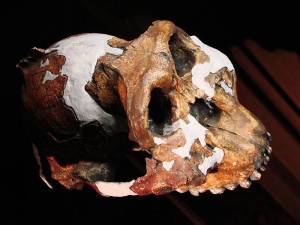
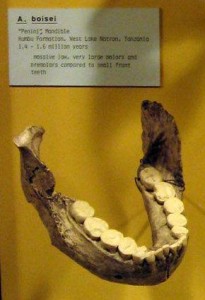
In the first course that I took in physical anthropology, I was most fascinated by the Paranthropus boisei face from Olduvai Gorge (see Figures 18.1 and 18.5) and the Natron/Peninj mandible from the Peninj site near Lake Natron. I still remember the first time I saw them, and the species has always been for me one of the more interesting discoveries in paleoanthropology. Their faces, jaws, and cheek teeth were massive and truly unforgettable. While the Olduvai material is attributed to Mary Leakey, it was her husband Louis who announced to the world that he had his “man.” He had been finding tools of what are now termed the Oldowan tradition and was hoping to discover who had made them when Mary discovered the first P. boisei fossils. They were assigned to a new genus and species: Zinjanthropus boisei, or “human from East Africa.” “Zinj” is a derivation of the archaic term “Zanj” for an area of the East African coast, and Boise was the surname of one of the Leakeys’ financial supporters (Wikipedia contributors 2015g). Louis claimed that they had their toolmaker, but many did not agree with his conclusion. They thought he had found a big, dumb, herbivorous, bipedal ape that would not have had the cognitive abilities to produce stone tools. Hand bones have been attributed to the species by some researchers. If they are correct in their assignment, the species may have been capable of tool manufacture and efficient use. However, while their brains were somewhat larger than earlier species, we still do not know if they had the cognitive abilities and thus whether tools from East African sites can be attributed to them.

Fossils from more than 100 individuals have been recovered in the last 55 years. Over time, the genus has changed from Zinjanthropus to Australopithecus to Paranthropus, but some researchers are still using genus: Australopithecus.
PHYLOGENY
Support for P. boisei being descended from Au. aethiopicus has steadily increased. However, some still group P. boisei as a sister species of P. robustus and believe that they descended from Au. africanus. The two more derived forms share a molar trait with Au. africanus, in that the second molar (M2) is larger than the third (M3). Members of the latter group, such as Henry McHenry, contend that the more primitive Au. aethiopicus is an example of homoplasy or convergent evolution, making the robust traits homoplasies. Paleoanthropologists have tended to be conservative in their acceptance of homoplasies; common ancestry is more parsimonious.
Except for the possible Au. aethiopicus → P. boisei scenario, the robust australopiths were evolutionary dead-ends as far as we know.

DISCOVERY AND GEOGRAPHIC RANGE
Mary Leakey discovered the first material in 1959 at Olduvai Gorge, Tanzania (see Figure 18.2). Nicknamed “Nutcracker Man,” “Zinj,” or “Dear Boy,” the skull and face were dated to 1.7 mya. Fossils attributed to the species have since been found at other sites in Tanzania (Peninj), Kenya (Chesowanja, West Lake Turkana, and Koobi Fora in the East Lake Turkana area), and Ethiopia (the Shungura Deposits and Konso). Richard Leakey discovered the Koobi Fora fossils. Thus, like Au. afarensis, the species had a broad geographic range and survived for over a million years.

PHYSICAL CHARACTERISTICS
While the robust forms are somewhat larger than the gracile forms, they do not differ much postcranially. It is their skulls that set them apart; P. boisei had the most pronounced masticatory adaptations, so that relative to the other two species, they are termed “hyper-robust.” Along with the other robust forms, they shared a buttressed skull, face, and mandible; large molars and premolars; a compound sagittal-nuchal crest (not compound in P. robustus); large muscles of mastication and nuchal muscles to support their heavy skulls; large, flared zygomatic arches; and a supraorbital torus to absorb the stress generated by chewing. According to some researchers, they shared the following with P. robustus and Homo: flexed skull base; more orthognathic face; lower face tucked under the braincase, thus increasing chewing force on the molars; a more parabolic dental arcade; and a longer thumb with broad, flat, distal phalanges that gave them better opposition and gripping power. The sagittal crest in P. boisei and P. robustus was more anteriorly positioned relative to Au. aethiopicus. In combination with less prognathism and the fact that their face was tucked under the braincase, the action and strength of the temporalis muscle was concentrated on the cheek teeth. The degree of robusticity and the size of craniofaciodental characteristics were unique to P. boisei. The zygomatics were large, heavy, and widely flared, making their faces very broad and the temporal fossa (the space between the zygomatic and the temporal bone) very deep. Again, this facilitated the passage of the temporalis muscle to insert on the mandible and expanded the attachment and anchoring of the masseter muscle. The mandible was massive, with a very robust and deep body and tall rami (vertical side portions), and the temporomandibular (jaw) joint was exceptionally large. Their front teeth were so dwarfed by their enormous cheek teeth, they almost look juvenile. Their premolars were molarized, and some had a third root. The third molar exhibited a unique wrinkling pattern. Their megadontia quotient (MQ) was 2.7, meaning that their teeth were 2.7 times larger than would be expected. P. boisei had the largest supraorbital torus of the robust forms. They were somewhat more encephalized than past species, with a cranial capacity of 514 cc (range = 494–537 cc). Like all australopiths, the species was sexually dimorphic, with males at 4’6″ (137 cm) tall and 108 lb (49 kg) and with more pronounced sagittal-nuchal crests and females at 4’1″ (124 cm) and 70 lb (34 kg).
Review of Derived Characteristics
- Same robust characteristics as seen in Au. aethiopicus with the following differences:
- More robust craniofaciodental characteristics.
- Sagittal crest more anteriorly placed.
- Large supraorbital torus.
- Huge molars and premolars.
- Large, heavier mandible.
- Encephalized.
- Flexed skull base.
- More orthognathic face.
- Molarized premolars.
- Lower face tucked under braincase, maximizing chewing force on molars and premolars.
- More parabolic dental arcade.
- Longer thumb with broad, flat distal phalanges.
ENVIRONMENT AND WAY OF LIFE
While they still had relatively long arms, one seldom hears anything about the arboreal habits of the robust forms. In East Africa, that is not too surprising since the forests had receded and the grasslands had expanded. By 1.8 mya, deep sea cores reveal an increase in the sahel type of environment, i.e. the area south of the Sahara that consists of dry grasslands and scrub forest. Based on microwear evidence from their molars, all or most of which are ground flat, P. boisei likely subsisted on open, dry-adapted plants. Silt on terrestrial foods would have contributed to the wear patterns. The availability of high-quality foods must have gotten even worse by the time of P. boisei, because their chewing and bite-force capabilities were increased relative to Au. aethiopicus. They ate a high proportion of C4 plants, i.e. open and more dry-adapted grasses and shrubs. It is thought that they likely survived on hard, tough, and brittle fallback foods during periods of preferred resource scarcity. This is another possible example of niche partitioning, in that sympatric Homo species may not have been able to utilize or digest either food category. Isotopic analyses to calculate the strontium-to-calcium ratio in fossilized bones of Au. africanus and P. robustus have determined that they ate some animal matter. It is possible that P. boisei did as well. While we do not know if they were scavenging or hunting animals, it is very possible that early hominins ate insects and insect larvae. All extant great apes consume social insects (ants and termites), and they derive a surprising degree of nutritional value from them. In traditional human societies, larvae are a favored resource.

It seems likely that P. boisei lived in small groups … or did they? Living in a more open environment in equatorial Africa, there would seemingly have been safety in numbers. Since they were adapted to low-quality savanna resources and could fall back on food items that were unavailable to other sympatric species, they may have avoided strong within- and between-group feeding competition. We also know that pair-bonding may have been necessary, as females were not as self-sufficient as their quadrupedal ancestors. They had babies that could not hold on and were surrounded by males that could rape or commit infanticide. There is an argument that monogamy is a male adaptation that increases paternity assurance while reducing the risk of infanticide. We know that males compete for mates, and the high degree of sexual dimorphism in P. boisei supports that fact. With all of the preceding arguments in mind, is it possible for apes with a tendency toward monogamy to live together without all of the rules and laws seen in modern humans to keep male competition and jealousy in check? While we could argue that they also could have lived in pairs with their dependent offspring and never ventured far from trees, how did females climb with their infants? We still have many unanswered questions about hominin behavioral ecology!

