Part IV: Pleistocene Epoch
21. Australopithecus sediba
Australopithecus sediba (~2.0 mya)
(“Southern ape / “fountain or wellspring” in the Sotho language)

SITES
Malapa, South Africa
PEOPLE
Lee and Matthew Berger and subsequent colleagues
INTRODUCTION
My research on this interesting species showed me how little I knew! Since (1) many biological anthropologists who teach human evolution are not paleoanthropologists, (2) Au. sediba is a fairly recent discovery, and (3) many textbooks have not as yet included much information about it, I have provided a more in-depth overview of this species.
Six well-preserved individuals of a new species of Australopithecus were discovered, beginning in 2008, at the cave site of Malapa, South Africa. Lee Berger’s crew is credited with the discovery after Berger’s nine-year-old son Matthew (see Figure 21.2) happened upon the fossils of a juvenile male (MH1) that became the holotype for the species (see Figure 21.1). The other five individuals were an adult male, an adult female (MH2) and, remarkably, an infant. It is of great utility in terms of interpreting morphology that much of the fossil material was found in situ (i.e. where the body settled versus missing or scattered about), demonstrating that they became buried and began fossilizing fairly rapidly. It is thought that they fell into a cavern, possibly lured by the presence of water in that then dry environment.

PHYLOGENY
Since Au. sediba shares characteristics with both Au. africanus and Homo, it is thought to possibly be intermediate between the two species. That scenario, of course, disagrees with Au. afarensis as being our direct ancestor, with Au. africanus as a side branch. According to Berger et al. (2010), Au. sediba is more distinct from Au. africanus than the latter is from Au. afarensis in hand, pelvis, foot, and ankle morphology. However, the team working with the material believe it should stay within the genus Australopithecus because it lacks the Homo encephalization trend. Surprisingly, Au. sediba is more similar to Homo erectus in some respects than to Early Homo (H. habilis or rudolfensis). There has also been speculation that Au. sediba is a derived form of Au. africanus, but it is problematic in that the Malapa specimens are only 100 kya more recent than the youngest Au. africanus material. Furthermore, because the Malapa material is contemporary with Early Homo material (H. habilis and rudolfensis), some insist that they cannot be ancestral to those species. However, Pickering et al. (2011) dispute that claim, since Early Homo material is still problematic, both taxonomically and spatiotemporally. Berger et al. (2010) have proposed four phylogenetic scenarios—that Au. sediba was the ancestor of Homo habilis, Homo rudolfensis, or Homo erectus, or was a sister species to the ancestor of the Homo lineage.

DISCOVERY AND GEOGRAPHIC RANGE
Because the “Malapa Party” (my term) discovery is such a great story, I used it to introduce the species and will not repeat it here. Malapa (see Figure 21.3) is 9.3 km northeast of Sterkfontein and 45 km north-northwest of Johannesburg in the Cradle of Humankind World Heritage Site (see Figure 21.4) (Wikipedia contributors 2015f). No fossil material has been found elsewhere.
PHYSICAL CHARACTERISTICS
In general, the species’ morphology is a mosaic of australopith- (especially Au. africanus) and Homo-like characteristics, but there are multiple lines of evidence to support its classification as a separate species (Berger et al. 2010). Since the Malapa material provides some of the best in situ evidence for many body parts, relative to other contemporary and previous species, I will spend more time discussing the physical and biomechanical significance.
The brain of Au. sediba was australopith-like in its size and “convolutional patterns” (Carlson et al. 2011). However, derived aspects of the frontal lobe show that Homo-like reorganization preceded pronounced encephalization (Carlson et al. 2011). This is an exciting discovery, in that we do not see so much of a gradual increase in size but rather that the hominin brain evolved in a mosaic fashion and that an increase in the association region was favored at the time. If cranial capacity alone was all that was available to infer cognition in Au. sediba, that important fact would be missed.

Like most areas of the body, the cranial morphology reflects australopith- (especially Au. africanus) and Homo-like characteristics (Berger et al. 2010). Relative to Au. africanus, Au. sediba had smaller teeth and a less robust face (see Figure 21.5). Of interest is that they are described as having an “incipient” nose, i.e. the beginnings of a protruding nose (Berger et al. 2010). They share various mandibular and dental characteristics with Au. africanus, Early Homo, and Homo erectus (Irish et al. 2013; de Ruiter et al. 2013). Mandibular size and shape and tooth size most closely resemble Early Homo, and while some tooth measures and growth pattern are most similar to H. erectus, the overall growth trajectory is unique compared to other fossil hominins (de Ruiter et al. 2013). Analysis of tooth shape, molar cusp patterns, and root number of the various teeth shows that Au. sediba and Au. africanus share five synapomorphies (shared traits among related species), thus supporting a South African australopith clade. In addition, Au. sediba shares five synapomorphies with H. habilis/rudolfensis/erectus, suggesting a South African australopith ancestry for our lineage (Irish et al. 2013).
Au. sediba retained relatively long arms and elevated shoulder joints for climbing (see Figure 21.6) (Kivell et al. 2011; Schmid et al. 2013). Except for three wrist bones and the distal phalanges of the four fingers, the right distal forearm, wrist, and hand have been recovered intact for the adult female MH2. While the morphology of the hand is unique in some ways and they were still capable of strong flexion for climbing, some traits are associated with tool production in Homo species, especially the longer, more dexterous thumb and shorter fingers that facilitate our strong precision pinching. The only other hand that is well preserved from the late Pliocene is that of OH 7, attributed to Homo habilis. However, the Malapa hand was better suited to tool production and there is still speculation that OH 7 was actually P. boisei, a robust australopith not in our lineage. Thus, Au. sediba may have been responsible for the tools that have been recovered at South African sites (Kivell et al. 2011).
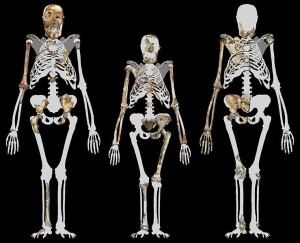
While determining the thoracic shape of fossil hominins has been a problem due to the lack of intact specimens, it is generally thought that australopiths had a conical thorax like extant great apes. A conical thorax is involved with arboreal locomotion, whereas our barrel-shaped thorax is (1) adapted for endurance walking and running, (2) related to shoulder breadth and arm-swinging to counter trunk rotation and facilitate our greater respiratory abilities, and (3) facilitated by a decrease in gut volume related to encephalization and a less herbaceous diet (first reviewed in Bramble and Lieberman 2004 and later in Schmid et al. 2013). The Au. sediba specimens support the notion of a conical shape to the upper thorax, but surprisingly, the lower thorax is not as flared as was previously thought, being more human-like. Schmid et al. (2013) assert that the “uncoupling” of the upper and lower trunk morphology in Au. sediba argues against a physiological response, in favor of a biomechanical one. The conical upper thorax would have been useful for climbing but would not have allowed for effective arm-swinging or heavy breathing and thus they were likely not capable of exertive walking and running. Their gut must have been smaller than a typical ape’s, suggesting a less herbaceous diet and setting the stage for the expensive tissue trade-off allowing for encephalization in our lineage (Schmid et al. 2013).
Au. sediba likely possessed the modern number of regional vertebrae. While the vertebrae of all hominoids increase in size from superior to inferior, earlier hominins lacked enlarged lumbar and sacral vertebral bodies and our pronounced sacral curve (see Williams et al. 2013 for references). Au. sediba is the earliest hominin to exhibit those characteristics, which are present in Homo erectus and all subsequent species. While the vertebrae in the five regions of our vertebral column have distinctive characteristics, they morphologically grade into one another so that there are transitional vertebrae, e.g. the seventh cervical vertebra resembles the first thoracic vertebra and so on. Unlike humans, the transitional thoracolumbar vertebra in Au. sediba, H. erectus (based on the Nariokotome specimen), and possibly Au. africanus is 11 versus 12. Thus while vertebra 12 is rib-bearing, it also likely functioned to elongate the lumbar region, increasing lower back flexibility (Williams et al. 2013).
The innominates were australopith-like in dimensions involved with childbirth but exhibited buttressing and other characteristics of Homo, such as iliac shape and vertical positioning, shortened ischium, and so forth (Kibii et al. 2011). The proximal femur was australopith-like with a small head and long neck, but the bicondylar/carrying angle and insertion of the gluteus maximus were humanlike. The knee exhibits both australopith- and human-like characteristics. Like us, they were capable of fully extending their knees during the swing phase of walking (DeSilva et al. 2013).
The partially articulated ankle and foot elements present a unique combination of primitive and derived traits. Humanlike characteristics include the morphology of the distal tibia, Achilles tendon insertion (calcaneal tuberosity), possible valgus (medially inverted) knee indicative of the bicondylar angle, probable arched foot, etc. Some aspects of the bones are more australopith-like, e.g. more gracile calcaneus; and some are more ape-like, e.g. robust medial malleolus (distal fibula, what we think of as our medial ankle “bone”) and some joints and muscle attachment sites (Zipfel et al. 2011).
Like in apes, initial ground contact was via the heel and lateral aspect of the foot, so that the foot was inverted. They then hyperpronated the foot by transferring weight medially so that the ankle was inverted and the plantar surface of the foot rolled medially and became everted. Modern humans with hyperpronation have problems with increased stress and wear on the joints, and the same is evident in Au. sediba remains (DeSilva et al. 2013). Hyperpronation, along with a robust medial malleolus, flexible midfoot, and upper limb adaptations, was apparently an adaptive response to bipedal terrestrial locomotion and retention of arboreal climbing abilities (J.M. DeSilva, personal communication with author, 2014). As in the ardipiths, the divergent hallux likely aided in climbing and would have given them more of a tripod foundation to stabilize their upright stance when standing and walking (Lovejoy et al. 2009).
Taken together, the mosaic of lower limb characteristics suggest that Au. sediba may have practiced a form of bipedalism unique from australopiths, or that they were descended from a more terrestrial ancestor and they then reverted to a more semi-arboreal lifestyle (DeSilva et al. 2013).
Review of Primitive Characteristics
- Brain size and convolutions.
- Australopith-like growth trajectory of teeth and jaws.
- Climbing adaptations: elevated shoulder joint, long arms, and strong hand flexion.
- Conical upper thorax.
- Australopith-like femoral head and neck.
- Some primitive aspects of ankle and foot.
- Retention of semi-divergent hallux.
Review of Derived Characteristics
- Reorganization of the frontal cortex.
- Small teeth and jaws and less robust face.
- Homo-like tooth shape and growth pattern.
- Humanlike lower thorax.
- Increased manual dexterity and precision grip.
- Longer, more dexterous thumb.
- Shorter fingers.
- Enlarged lumbar and sacral bodies and sacral curve.
- Buttressed and more vertical ilium.
- Homo-like carrying angle and gluteus maximus insertion.
- Full knee extension.
- Humanlike aspects of tibia, ankle, and foot.
- Possibly unique form of bipedalism, e.g. hyperpronation.
ENVIRONMENT AND WAY OF LIFE
The habitat of Au. sediba is thought to have been a mosaic environment of wood- and grasslands. Phytoliths from their tooth enamel indicate that they had access to forest products. Phytoliths are species-specific silica bodies in plants that can be used to identify what plant species were consumed. Fruit, leaves, wood, and bark were identified, with the latter being a first for fossil hominins (Henry et al. 2012).
Unlike other australopiths and paranthropines, the diet of Au. sediba most closely resembled savanna-dwelling chimps and secondarily Ar. ramidus, in that they specialized on C3 plants, such as herbaceous plants, shrub and tree plant products, and grasses, and possibly animals that consumed those items. We know that C4 plants were available at the time because remains of those more open, dry-adapted plants have been recovered in the same layers within which the Malapa material was found. In addition, C4-consuming rodents, horses, and bovids were resident at the time. Their diet was thus less “carbonically” (I made that up for fun!) varied than contemporary hominin species inhabiting similar environments, such as Au. africanus and Paranthropus robustus, as well as Homo species that had an even broader diet. Microwear analyses show that the two Malapa individuals (MH1 and MH2) under study ate a greater percentage of hard food items relative to other australopiths, being more similar to P. robustus or H. erectus (Henry et al. 2012).
Being C3 specialists in a C4-dominated environment may have involved more time invested in foraging, or they may have favored a more closed environment for feeding. It is thus likely that there were enough woodland or riverine environments to have supported the population. Additionally, they could have consumed hard items when available or as fallback foods.

