Messages from STEM and Health Science Scholars
Writing in Science: Creating a Lab Write-Up
Article Excerpt with Reflection
Cesar Hernandez and Thawanhathai Kiatsutthakorn
Optimization of the Growth of Neuronal Stem Cells in Vitro
Introduction
The cerebellum (CB) is a part of the brain that functions in coordination and balance, and holds the most abundant amount of neurons, roughly 80%, of the human brain (Azevedo, 2009). Due to the proliferation of granule cell precursors (GCPs), the CB is able to grow the most during infant stages. The three layers found in the cerebellum are: molecular layer, granule cell layers, and purkinje cell layer (PCL). The PCL gives rise to purkinje cells, which are inhibitory neurons in the ventricular zone (VZ) of the CB. Purkinje cells are important because they release Sonic Hedgehog, a signal that is responsible for cell differentiation, especially post injury to the brain. Progenitor cells that express the neural stem cell marker Nestin, known as Nestin expressing progenitors (NEPs), are typically found in the purkinje cell layer; however, recent studies have shown (Wojcinski, 2017) that after granule cell ablation in the external granule layer (EGL) resulting from traumatic brain injury, NEPs migrate to the EGL and become granule cells instead of becoming purkinje cells in the PCL. The abnormal expression of the Atoh1 gene responsible for neuron differentiation (Mulvaney, 2012) in the VZ can convert VZ neurons to be descendants of the upper rhombic lip, which is the area that produces granule cells (Yamada, 2014). The migration of NEPs are a crucial component of post-injury cell behavior, and has been known to be mediated by sonic hedgehog signaling. Thus, experimenting with in vitro cell culture using neurons derived from mice can give further insight on this pathway, and whether or not the pathway is only effective in an in vivo environment.
The proposed experiment of how Nestin expressing progenitors could grow in culture to mimic in vivo behavior can be observed by using monolayer cell culture, TUNEL cell death assay, fluorescence microscopy, and live imaging. It is hypothesized that NEPs grown in vitro could mimic the behavior of those grown in vivo.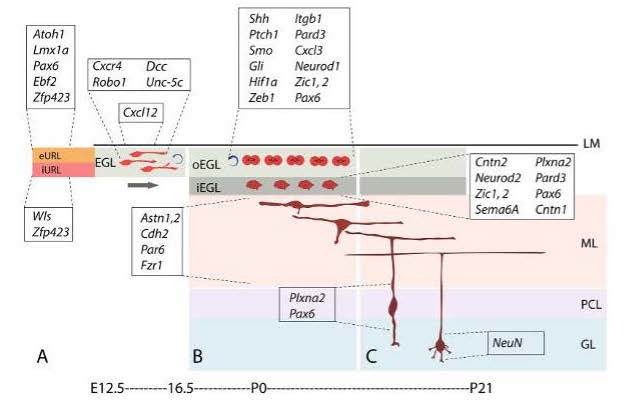
Procedure
Cellular behavior found in vivo must be replicated in vitro
The main objective is to figure out how to grow cells in a monolayer culture. This is a culture technique that involves the use of a substrate to create a surface. The cerebellar cells will be used in the implementation of this technique, and the use of trypsinization to allow adherent cells to stick to the surface of the culture will be used to test for the survival of cells in the cell environment that is trying to be replicated. The media that will be used is Dulbeco’s Modified Eagle Medium (DMEM). Cells will be incubated after the procedure and will be monitored every 72 hours. Successful implementation of this technique will examine the cells as they grow to make sure they are mimicking the cell behavior found in vivo. Once cells are functioning as they do in the cellular pathways of a healthy brain, the concentration is shifted to the implementation of a post-injury cell environment.
Cells ablation after traumatic brain injury must be proven
In order to recreate a post-injury environment in vitro, there must be an injury sustained to the cerebellar tissue. To do this, mice were exposed to irradiation at P1. To prove that cell ablation was in fact occurring after the occurrence of injury, the TUNEL cell death assay technique was used. This assay is used for confirmation when it is presumed that cells are experiencing apoptosis. When there is cell death, one of the key characteristics that is happening is DNA fragmentation. When this happens, an enzyme called TdT(Terminal transferase) migrates to the fragmented pieces of DNA and adds fluorescently labeled nucleotides(dUTP) to them. This way the dead cells are able to be seen using microscopy.
Monolayer cell culture must be able to sustain cells
Besides using a TUNEL cell death assay to prove that TBI results in apoptosis, it is also used to make sure that cells are able to survive in the monolayer cell culture. The culture serves the purpose to act as an environment where cells can be sustained and maintain a healthy growth pattern. If the death rate of the cells is too large then the in vitro environment is inefficient to observe migration and differentiation of the cerebellar cells.
The migration of NEPs into the EGL is observed using fluorescence microscopy.
The migratory patterns of NEPs must first be observed to gain insight and confirmation that cells are in fact moving from the PCL into a foreign brain region, specifically the EGL. To see the migratory patterns of the NEPs immunohistochemistry proves to be efficient in that it allows for differential properties of the cells in focus. Green fluorescent protein(gfp) was the cell marker used to track the NEPs. The gfp protein was attached to the NEPs in the PCL by binding to the DNA of the individual cells. As long as there is oxygen present in the cellular environment the protein has the ability to fluoresce due to the three amino acids- Serosine 65, Kerosene 66, Glycine 67- found in the helix of the structure. No further action is required to maintain fluorescence of gfp. Gfp fluorescence was used further to track different proteins such as PAX6, SOX2, and Ki67 so that observation of a possible change in gene expression during migration could be visualized. This way, once migration is observed, the differentiation of the NEPs in the EGL can also be observed.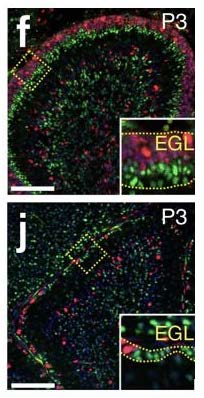
Cellular activity in vitro must be visualized
The use of immunofluorescence allows for better visualization of the cells in an in vitro environment. Once the cells are visible in the cell culture the use of an inverted microscope provides further aid in the examination of the cell activity. Periodic imaging of the cells under the microscope allows for time lapse videos of live cell behavior in the cell culture. This is important because not only does it help in the acquired results of a particular experiment, it also allows one to check if the correct behavior is occurring in the cell culture or if the in vivo environment failed to be replicated in vitro. This is characterized by different cell growth, expansion, and movement in the culture than what is typically observed in vivo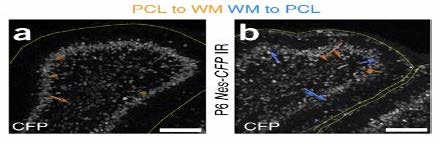
Discussion
Before it can be established whether or not NEPs are able to mimic the in vivo behavior outside of the live mice, it must be determined that NEPs are able to grow in a cell culture. After performing the monolayer cell culture, it is expected that NEPs can grow in an in vitro environment. With this growth, the TUNEL cell death assay will show that these cells could, in fact, be damaged. This would develop a baseline for the observation of migration in NEPs to the damaged area for regeneration. By using dsRed coral discosoma sp, mutated gfp (citrine, mcfp) as fluorescent markers and time lapse imaging, the migration pattern of these cells could be observed and recorded. The NEPs are expected to migrate to the EGL to replenish damaged granule cells, as seen in the cerebellum of live wild type mice.
It is anticipated that understanding the migration pathway, and how the cerebellum is able to repair damages during developmental stages in infants can contribute to the prevention of cerebellar hypoplasia which is a risk factor for autism (Wang, 2014).
A research study (Miller, 2008) has uncovered new pathways that can also be held accountable for the migratory patterns of NEPs to the EGL, such as the CXCR4/CXCL12 chemoattractant pathway. The cells that cover the surface of the EGL have SDF1 expression which is the ligand that the CXCR4 receptors in this pathway bind to. Therefore, by putting emphasis on the function of the receptors, the understanding of the chemoattractant signaling on the migration of NEPs during cerebellar regeneration can be enhanced. In the future, the SDF1-CXCR4 chemoattractant pathway can be investigated to understand how this pathway contributes to the migration of NEPs.
Reflection
As aspiring science professionals, it is imperative that we get experience working in the research field. It gives the opportunity to witness firsthand how today’s scientists make contributions to their field of expertise by addressing the gaps in knowledge, brainstorming ideas to dive into those unknown areas, and using the newfound information to expand basic and applied knowledge. The specific experiment that we were presented the opportunity to take part in involves the investigation of the regenerative capacity of the cerebellum after traumatic brain injury. Working on this project let us develop a skill that is often overlooked by many young scientists: writing scientific papers using proper language in order to summarize background information, experimental planning, procedures, results, and the significance of the findings.
Having to create a lab write-up is a very unique experience due to the fact that one cannot just decide to type one up on any day that they feel like it. First, one must gain experience reading empirical articles relating to the subject of study in order to gain comfort with the complex vocabulary that is found within an article. Many times, the key words included in scientific writing are largely new to the reader experience in related fields of research. It can be compared to learning a new language: one has to learn the meanings of words to try to make sense of the reading material. Once one has gotten the hang of the language, one must decide how to use it. To explain this, just because scientists are comfortable with the language that they use, it does not mean that their audience will have the same level of fluency. Often, the information will have to be simplified in writing so that the reader can gain an understanding that matches the amount of knowledge in their possession.
While looking into the background and analyzing previous experiment results presented a lot of difficulty, it was still not the most challenging part of our research project; the most difficult obstacle to overcome was proposing methods to test our theory but not being able to perform any in an actual laboratory setting. We were unable to obtain the actual stem cells we wanted to investigate because of its exclusivity and price. We could not afford to take any risks mishandling the cells or damaging them accidentally. Not only were the cells out of our reach, the CDC and campus guidelines for the COVID-19 pandemic also restricted us from working in the laboratory at our university. Had we been able to work with the stem cells we were investigating and been able to perform our proposed methods in a laboratory, we would have had evidence to either support or refute our hypothesis. Because of this, our project could only be explained theoretically, not in actuality.
Even though we did not perform our experiments, we spent most of our time reading through previous studies to grasp an understanding of the behavior of NEPs. As previously mentioned, reading new research articles can be difficult due to a new and different jargon that we normally don’t use in casual conversations. As undergraduate students with little experience in the research field compared to the post doctoral researchers, adjusting to their language took a lot of time. We spent many weeks breaking down each figure, trying to dissect the experiments and understand what the results meant. We also had to find the aim or purpose of the experiment; we had to figure out what questions the scientists were asking before we could fully understand the answers that they got. Learning any new language takes time, but because we were able to dedicate most of our project to understanding previous studies, coming up with new methods to test our hypothesis was easier than we expected.
This is a lab write-up of our research that is meant for amateur scientists with above-average knowledge or non-science college students. The introduction, procedures, and discussion all aim to use the correct scientific names for the cells and anatomical structures that we focused on for our research, while also giving a detailed, yet not overwhelming, explanation of the expectations and interpretations. We tried to avoid going too deep into any advanced explanations of the occurrences expected to be found in the post-injury cellular environment of our proposed experiment, so that a casual reader with a basic, or even slightly below basic, understanding of neuroscience could follow along with our write up. We also explained our proposed laboratory material to paint a clearer picture of the steps we were planning to take in our research to test our hypothesis. This write-up also gives the reader a basic understanding of the theory behind the experimental tools, and it allows for the possibility to brainstorm about when a scientist may use these tools in other experiments relating to different cellular pathways or different anatomical structures altogether.
After spending hours gathering background information on cellular processes, biochemical tools, and experimental procedures, the work ended up paying off, as it resulted in the communication of scientific information. While reading our proposed study on the behavior of nestin expressing progenitor cells in an in vitro environment, we encourage you to look up anything that you may not understand, or are curious about, whether that may be a single word, a study that we reference, or any structure that you want to gain more knowledge about. Perhaps most importantly, you may want to dive deeper into the background of nestin-expressing progenitors by reading one of the referenced studies, if time allows. By doing this, even though you may not feel like you are doing so, you are thinking about the reading as an intuitive scientist would. If you want to take it a step further you may even want to think about how this research can be expanded in future studies.
References
Azevedo, F.A. et al. Equal numbers of neuronal and nonneuronal cells make the human brain and isometrically scaled-up primate brain. J. Comp. Neurol. 513, 532–541 (2009).
Consalez, G. G., Goldowitz, D., Casoni, F., & Hawkes, R. (2021). Origins, Development, and Compartmentation of the Granule Cells of the Cerebellum. Frontiers in neural circuits, 14, 611841. https://doi.org/10.3389/fncir.2020.611841
Lackey, E. P., Heck, D. H., & Sillitoe, R. V. (2018). Recent advances in understanding the mechanisms of cerebellar granule cell development and function and their contribution to behavior. F1000Research, 7, F1000 Faculty Rev-1142. https://doi.org/10.12688/f1000research.15021.1
Miller, R. J., Banisadr, G., & Bhattacharyya, B. J. (2008). CXCR4 signaling in the regulation of stem cell migration and development. Journal of neuroimmunology, 198(1-2), 31–38. https://doi.org/10.1016/j.jneuroim.2008.04.008
Mulvaney, J., & Dabdoub, A. (2012). Atoh1, an essential transcription factor in neurogenesis and intestinal and inner ear development: function, regulation, and context dependency. Journal of the Association for Research in Otolaryngology : JARO, 13(3), 281–293. https://doi.org/10.1007/s10162-012-0317-4
Yamada, M. et al. Specification of spatial identities of cerebellar neuron progenitors by ptf1a and atoh1 for proper production of GABAergic and glutamatergic neurons. J. Neurosci. 34, 4786–4800 (2014).
Wang, S.S., Kloth, A.D. & Badura, A. The cerebellum, sensitive periods, and autism. Neuron 83, 518–532 (2014).

