Learning Activities 5.1 – 5.3: Equilibrium Vapor Pressure of Water
Summary of Learning Activities
Learning Activity 5.1: Equilibrium Vapor Pressure of Water at Room Temperature
Learning Activity 5.2: Effects of Boiling Water on Pressure and Temperature in a Vacuum Chamber
Learning Activity 5.3: Effects of Water Temperature on Equilibrium Vapor Pressure
- Measuring the equilibrium vapor pressure of water for different water temperatures.
- Collect data to qualitatively assess water’s changing equilibrium vapor pressure changes in the temperature of the liquid water.
- Recognize that once the pressure at which the boiling point of water occurs, achieving lower pressures in the vacuum system corresponds to the decreasing temperature state of the water.
- Report calculated values with appropriate significant figures.
Suggested Pre-lab Assignment
-
Use an internet resource to determine the equilibrium vapor pressure of water at the following temperatures: 45°C, 20°C, 3°C. Suggested Sources:
- View Video 5.1 about boiling water inside the vacuum chamber:
https://youtu.be/h1YAP7XkzEc?si=zgkfgOjevciMc_C6Video 5.1. Boiling and Freezing Water in a Rough Vacuum System by Vacuum Demos, https://youtu.be/h1YAP7XkzEc?si=q-iiZ0TItaJDOKeQ. - Why did the water start boiling when pressure was reduced in the chamber?
- What happens to the temperature of the water if the pump-down process is allowed to continue after boiling starts? What happens to the energy of the water molecules? Why?
- Did you observe any phase transitions during the continuous pump-down process? If yes, explain why you think the water went through this phase transition.
Theoretical Background
There are two opposing processes working at the interface of liquid and gas at the same time, evaporation of molecules from the surface to the vapor phase and condensation of those molecules back to the liquid phase. When the two opposing processes are equal, the equilibrium vapor pressure of the substance has been reached. Equilibrium vapor pressure depends on the temperature of the substance and is described by the following expression:
[latex]\begin{equation} log_{10}P_{vp} \thinspace = \thinspace A \thinspace - \thinspace \frac {B}{T} \end{equation}[/latex]
where
Pvp is the vapor pressure in torr,
A and B are constants for a specific gas, and
T is the temperature of the substance in Kelvin
When the temperature of the substance increases, its equilibrium vapor pressure also increases. This relationship is shown in Figure 5.1. Conversely, as the temperature of the substance decreases, its equilibrium vapor pressure also decreases.
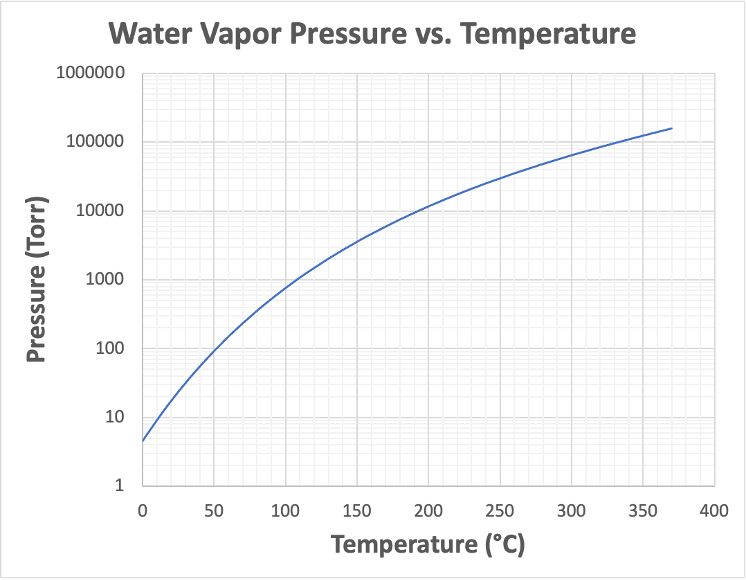
If a volume of liquid water is placed in a vacuum chamber and the pressure within the chamber is subsequently reduced, the liquid water will start to boil when the pressure in the chamber reaches the equilibrium vapor pressure of the water. Under a nominal atmospheric pressure condition of 1 atm, which is approximately 760 Torr, water boils at 100°C. This temperature is called the normal boiling point of water. As the air pressure decreases, the amount of energy needed by the water molecules for them to escape from the liquid state decreases. Using a vacuum system to pump down to lower pressure conditions makes it possible for us to observe how the equilibrium vapor pressure changes with different water temperatures.
Equipment and Materials
- Rough Vacuum Equipment Trainer (RVET)
- Omega Thermometer HH911T (or similar device)
- Device for heating water
- 2 or 3 glass containers (one glass container must fit inside the RVET chamber and NOT completely block the outlet hole in the base plate)
- Oven mitt to handle hot glass container
- Water and ice
Procedure
Learning Activity 5.1: Equilibrium Vapor Pressure of Water at Room Temperature
- Pour water into two glass containers, each approximately 1/4 full. Allow time for the water temperature in each container to reach approximately room temperature.
- Put one of the glass containers with room temperature water into the RVET. If a thermocouple temperature measurement device is available, immerse the probe in the water. Apply power to the temperature recording device.
- Place the bell jar over the glass container.
- Record the temperature of the water (T1) in Table 5.2.
- Record the pressure value on the capacitance diaphragm gauge (P1_P) in Table 5.2.
- Make sure the RVET vent valve is closed.
- Turn on the pump. Open the valve between the chamber and the pump.
- When the water just starts splashing in a boil, close the valve between the chamber and the pump.
- Record the temperature of the water (T2) in Table 5.2.
- Record the pressure value on the capacitance diaphragm gauge (P2_P) in Table 5.2.
- Vent the system.
- Remove the glass container from the RVET.
Table 5.2. Vapor Pressure Data at Room Temperature Water.
Date data collected: _________
|
Temperature of the water (T1) |
|
|
Chamber pressure (P1_P) |
|
|
Temperature of the water (T2) |
|
|
Chamber pressure (P2_P) |
|
Question:
If you are/were able to stick your finger in the beaker to experience how hot or cold the water feels, do you expect it to be very hot? Remember, it was just boiling in the chamber. Explain your response.
Learning Activity 5.2: Effects of Boiling Water on Pressure and Temperature in a Vacuum Chamber
- Place the second glass container containing room temperature water in the RVET.
- Before pumping down, record the pressure from the capacitance diaphragm gauge and the temperature from the thermometer on the first row (initial conditions).
- Record the temperature of the water when the water starts splashing in a boil and record the pressure value on the capacitance diaphragm gauge in Table 5.3.
- Start a stopwatch.
- Continue to pump the chamber.
- After 15 seconds, record the temperature of the water and record the pressure value on the capacitance diaphragm gauge in Table 5.3.
- Record temperature and pressure measurements every 15 seconds 11 more times for a total of three (3) additional minutes of pumping on the system after the water started boiling. NOTE: Does the water continue to boil?
- Close the valve between the chamber and the pump.
- Vent the system.
Table 5.3. Pumping on Room Temperature Water after it boils.
Date data collected: _________Site 1:
Site 2: Spring 2020, Wichita Falls, TX
(continued to pump down)Site 3: Spring 2023, Bloomington, MN
(continued to pump down)Time (seconds)
Chamber Pressure (Torr)
Water Temp (°C)
Chamber Pressure (Torr)
Water Temp (°C)
Chamber Pressure (Torr)
Water Temp (°C)
Initial Conditions
744
19
719
21.9
0
17.5
17.3
19.0
21.7
15
17.1
16.9
18.4
21.6
30
16.9
16.5
18.3
21.3
45
16.6
16.1
18.3
21.3
60
16.3
15.7
18.3
21.2
75
16.1
15.5
18.3
21.2
90
15.8
15.1
18.3
21.2
105
15.4
14.8
18.2
21.2
120
15.2
14.6
18.3
21.2
135
15.0
14.2
18.2
21.2
150
14.9
14.0
18.2
21.2
165
14.5
13.7
18.0
21.2
180
14.3
13.5
18.3
21.1
Learning Activity 5.3: Effects of Water Temperature on Equilibrium Vapor Pressure
- Remove the glass container from the RVET. Pour off some of the water in the glass container. Add ice. Let the ice water mix sit for two minutes.
- Repeat Steps 2 – 11 using the container with ice water.
- Remove the glass container from the RVET.
- Use a device to heat some water to a temperature between 150 – 175°F. Pour the hot water into a glass container.
- Repeat Steps 2 – 11 using the container with heated water. Use an oven mitt to transfer the glass container with hot water and place it in the RVET.
- After the system is vented, use an oven mitt to remove the glass container from the RVET.
Table 5.4. Temperature Dependence on Vapor Pressure.
Date data collected: _________Site: Normandale
Cold water
Room temperature water
Hot water
Temperature of the water (T1)
Chamber pressure (P1_P)
Temperature of the water (T2)
Chamber pressure (P2_P)
Activity Wrap Up:
- Make sure the beaker with water is removed from the RVET.
- Use a wipe to dry the inner surfaces of the chamber.
- Pump down the RVET until the pressure within the chamber is less than 10 Torr; pump on the empty RVET system for several minutes allowing the system to remove additional water vapor molecules.
- Close the valve between the pump and the chamber leaving the chamber under vacuum.
- Power off the RVET system.
- Power off the water heating device and discard remaining hot water.
- Discard the ice water.
- Dry and store glass containers.
- Put away other materials in designated locations.
Analysis of Results:
- Create two separate graphs with data from all three sites included: one plotting the chamber pressure data versus time and the other plotting water temperature data versus time collected in Steps 13 – 21. The first point on both graphs should be the zero-time measurement (when the stopwatch was started).
- How did the results for water vapor pressure of room temperature water compare from site to site?
- How does the water vapor pressure data for different temperatures of water recorded during the in-class activity compare to expected values? You may use the table at the following link: https://en.wikipedia.org/wiki/Vapour_pressure_of_water.
- Will the results for the equilibrium vapor pressure of water change if the atmospheric pressure condition is different?
- What is the Boiling Point temperature of water in Salt Lake City, UT? What does this mean for processes like boiling food?
- Which of the site locations is likely to experience a Boiling Point temperature of water closest to water’s Normal Boiling Point Temperature and why?
- What happens to the temperature of the water after it starts to boil and the pressure of the chamber continues to fall? Does the water appear to boil continuously after it starts boiling?

This work is supported by the National Science Foundation under grant number 2000454. Any opinions, findings, and conclusions or recommendations expressed in this e-book are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Media Attributions
- Figure 5.1. © E. Brewer is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license

